Fujifilm Introduces CAD Eye: AI-Powered Endoscopy System to Revolutionize Colonoscopy Procedures
- by dgihost.com
Fujifilm’s CAD Eye: AI-Powered Endoscopy System for Advanced Colonoscopy
Fujifilm has unveiled its latest innovation in the field of endoscopic imaging with the introduction of CAD Eye, an AI-powered detection system designed to revolutionize colonoscopy procedures. The FDA recently granted 510(k) clearance for CAD Eye, marking a significant milestone in the advancement of medical technology.
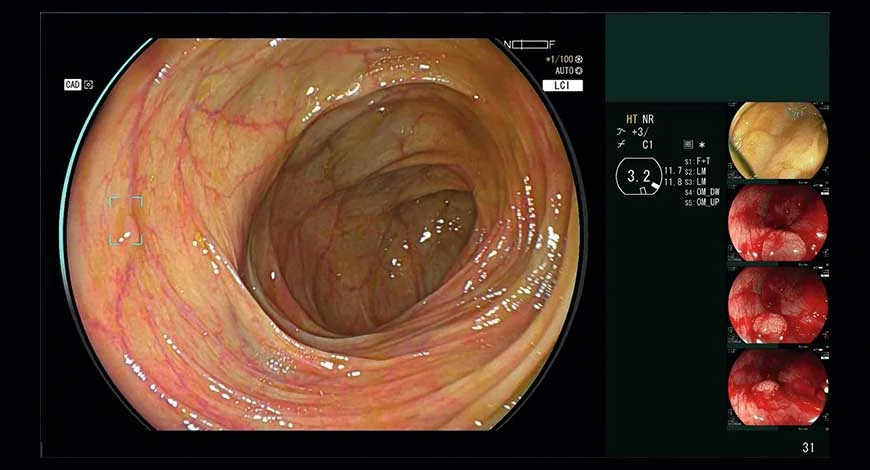
CAD Eye is engineered to enable real-time detection of colonic mucosal lesions, such as polyps and adenomas, during colonoscopy procedures. By leveraging artificial intelligence, this cutting-edge system supports endoscopists in accurately identifying and removing pre-cancerous lesions, irrespective of their size, shape, or color. This groundbreaking technology enters a market currently dominated by Medtronic and its GI Genius system, offering a promising alternative for healthcare professionals and patients alike.
One of the key features of CAD Eye is its compatibility with both white light imaging and Linked Color Imaging (LCI), providing enhanced visualization capabilities for improved mucosal visualization. Additionally, Fujifilm has incorporated an enhanced visualization mode to differentiate the red color spectrum, further enhancing the system’s effectiveness in lesion detection.
Anthony Borrelli, executive director of endoscopy product & marketing at Fujifilm Healthcare Americas Corporation, emphasized the significance of CAD Eye’s internal development and AI training. The system was trained exclusively on images captured by Fujifilm endoscopy imaging equipment, leveraging the company’s renowned expertise in optics and image quality.
Furthermore, CAD Eye offers seamless integration into physicians’ workflows, allowing for easy activation and deactivation directly from the endoscopic handle. This innovative feature streamlines the procedure and minimizes disruptions, enhancing efficiency and patient care.
The development of CAD Eye involved deep learning technology at Fujifilm’s Tokyo-based global AI technology center. Through rigorous validation using histologically confirmed polyps in clinical images, the system has demonstrated remarkable accuracy in detecting lesions that may be missed by conventional methods.
Clinical studies have shown that CAD Eye leads to significant advancements in colorectal cancer detection and diagnosis, detecting more adenomas during screening and surveillance compared to conventional colonoscopy without AI assistance. With its ability to detect colorectal neoplastic lesions at a level comparable to expert gastroenterologists, CAD Eye represents a pivotal advancement in gastrointestinal healthcare.
Dr. Sravanthi Parasa of the Swedish Medical Center expressed enthusiasm for Fujifilm’s AI CAD polyp detection algorithm, emphasizing its potential to enhance patient outcomes and improve precision in colorectal cancer detection. The FDA’s approval of CAD Eye underscores its importance in safeguarding patient health and reinforcing the commitment to embracing technological innovations in gastrointestinal healthcare.
Fujifilm’s CAD Eye: AI-Powered Endoscopy System for Advanced Colonoscopy Fujifilm has unveiled its latest innovation in the field of endoscopic imaging with the introduction of CAD Eye, an AI-powered detection system designed to revolutionize colonoscopy procedures. The FDA recently granted 510(k) clearance for CAD Eye, marking a significant milestone in the advancement of medical technology.…